Our Science
There are multiple underlying causes of Parkinson’s, so it’s important to take a personalized approach to treatment. We are using advanced tools like machine learning and genetic sequencing to identify individuals that are most likely to respond favorably to our investigational treatment, as well as translatable disease-relevant digital biomarkers to efficiently measure our clinical impact.

-
Leveraging Genetics to Identify the Right Patients
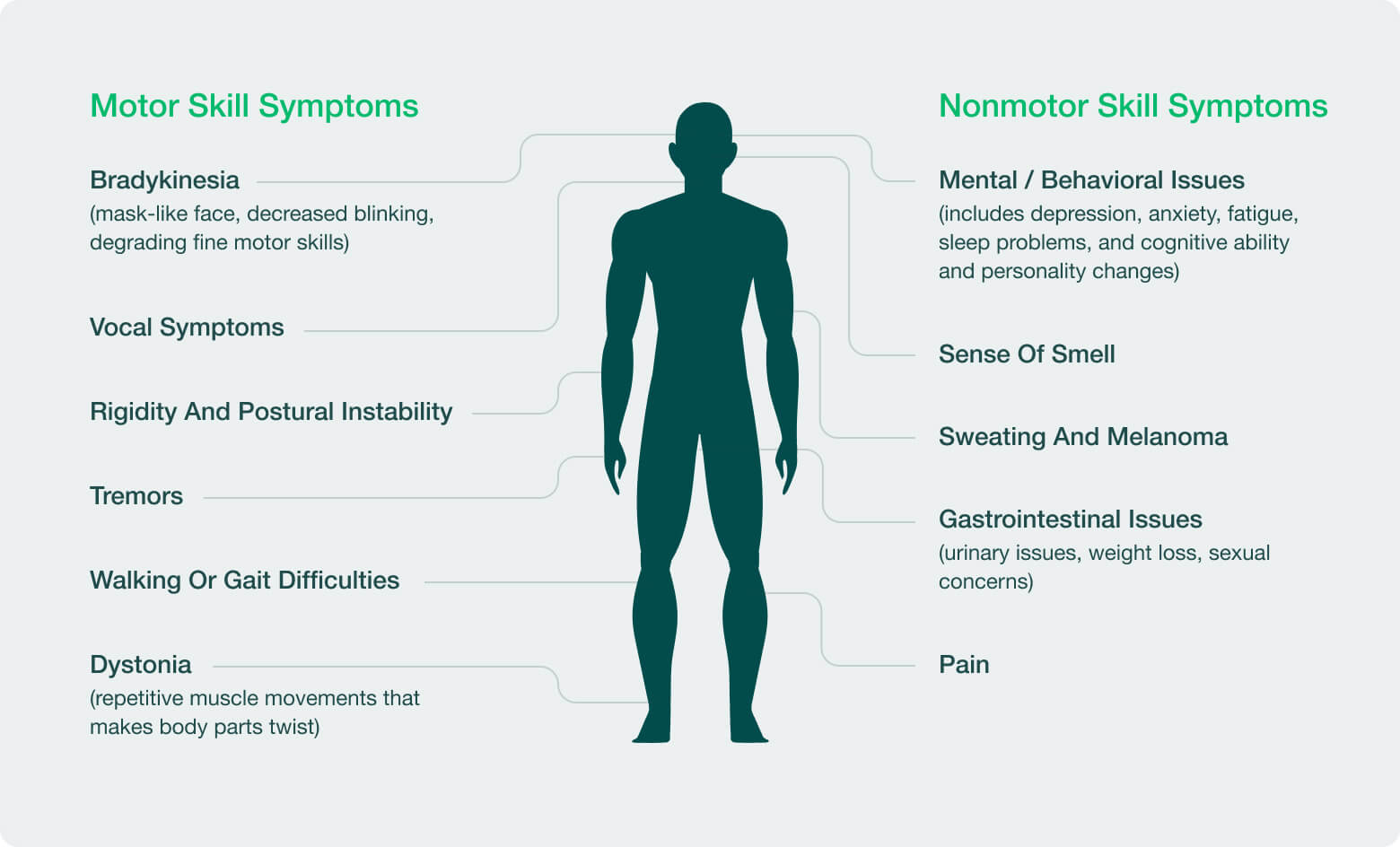
PD is the second most common neurodegenerative disease. The Parkinson’s Foundation estimates that nearly 90,000 people are diagnosed with Parkinson’s disease every year in the U.S., and that 1.2 million people in the U.S. will be living with PD by 2030. Today, however, there are no therapies available that impact the underlying progression of PD, and current treatment options are only used to alleviate symptoms. A core challenge in developing therapies that impact PD progression is that there are likely many causes of PD, and historically little progress has been made in pairing potential therapies with patients who are most likely to benefit.
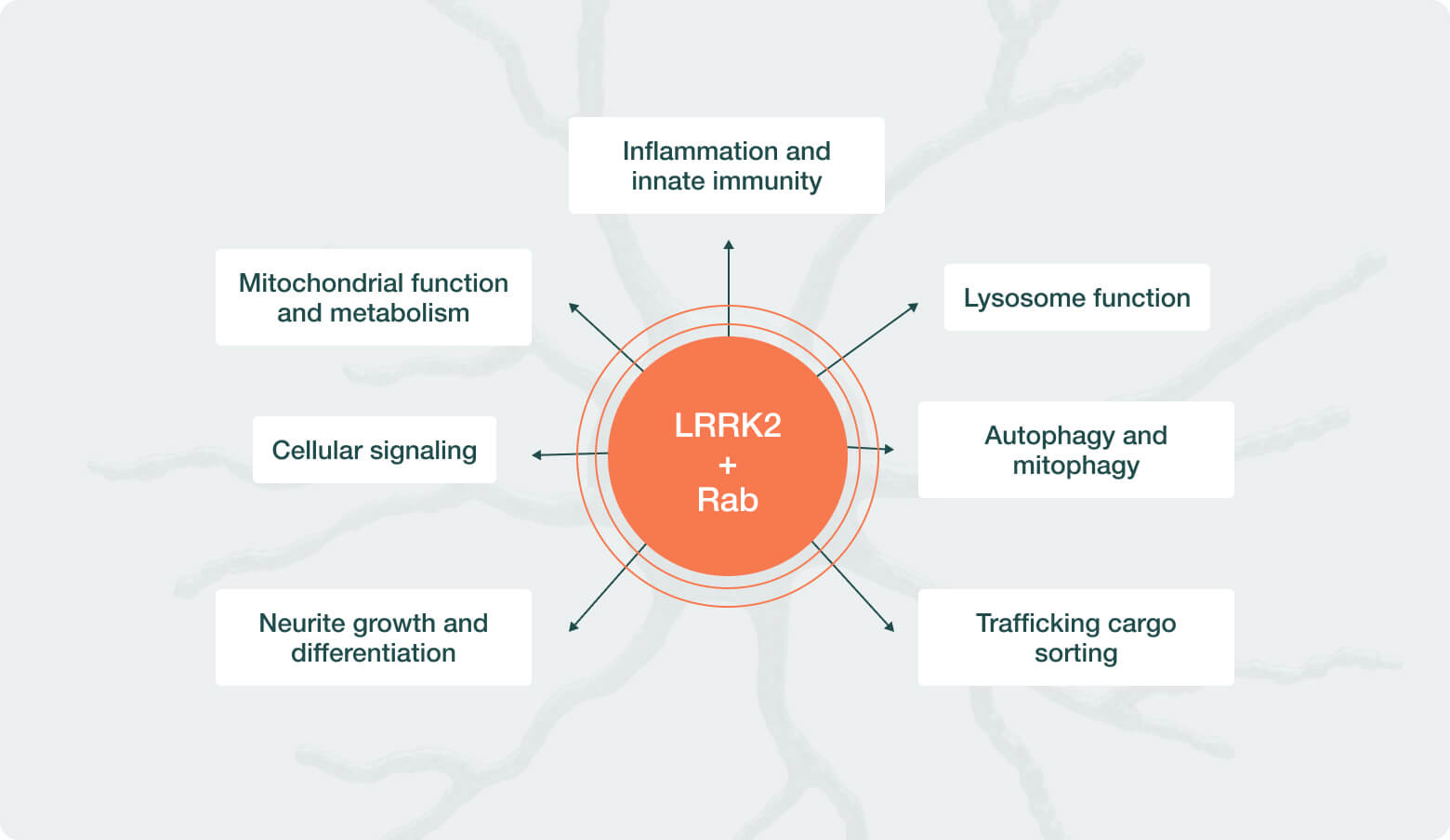
Neuron23 aims to change this paradigm with a precision medicine approach to PD. Our scientific co-founders are leaders in the fields of human genetics and machine learning, and bringing these two disciplines together has created the opportunity to identify genetic signatures that are correlated with increased activity in our target of interest, LRRK2. Learn more about LRRK2 here
To identify these unique, proprietary genetic signatures, we utilized advanced machine learning tools to deeply analyze many public and private PD data sets. Through this effort we identified single-nucleotide polymorphisms (SNPs) – common variations in an individual’s DNA – that are predictive of LRRK2 pathway overactivity and are present in over 30% of all people with PD. People with PD who have these SNPs, together with people who inherited rare LRRK2 gene mutations, make up the population we collectively refer to as LRRK2-driven PD and represent who we believe are most likely to benefit from LRRK2 inhibition.
We utilized these SNPs and a set of proprietary algorithms to build the first-ever companion diagnostic (CDx) for PD, developed in partnership with QIAGEN. Our CDx is a blood test that allows us to identify LRRK2-driven PD patients. We are now utilizing this CDx in our Phase 2 clinical trial, NEULARK (link), to select patients we believe are most likely to respond to our LRRK2 inhibitor, NEU-411.
-
Developing Best-in-Class Small Molecules
LRRK2 has historically been a difficult target to drug primarily due to the difficulty of designing small molecule kinase inhibitors that are not only potent and brain-penetrant but also highly selective, minimizing off-target effects. The chemistry team at Neuron23 has utilized deep expertise in computational and medicinal chemistry to develop a potent and selective inhibitor of LRRK2, NEU-411, with an exceptional therapeutic index. In a Phase 1 clinical trial, NEU-411 was safe and well tolerated in over 100 healthy volunteers for up to 28 days. NEU-411 also demonstrated robust target engagement of the LRRK2 pathway using fluid-based biomarkers that can be detected in healthy individuals, demonstrating NEU-411’s ability to inhibit LRRK2. Extensive pharmacodynamic, pharmacokinetic and chronic safety studies in animals have provided a strong foundation for NEU-411’s clinical development.
-
Innovations in Measuring PD Progression
PD progression has been historically measured using a series of rating scales, first developed in the 1980’s, that rely on Neurologist exams or the patient remembering and reporting their symptoms severity during clinic visits every 4-8 weeks during a clinical trial. This approach creates a burden on participants, while inadequately differentiating normal ups-and-downs that occur from disease progression.
We are taking a different approach by measuring PD progression in the NEULARK clinical trial by utilizing technologies like smartphones and smartwatches to measure symptoms much more frequently as individuals go about their day. The Roche PD Monitoring Application is an innovative digital biomarker developed by Roche Information Solutions (RIS), the Roche group focused on digital health solutions. Participants will use a smartphone equipped with proprietary software that frequently measures PD symptoms such as slowed movement and tremor, as well as non-motor symptoms such as cognition. By integrating an advanced digital biomarker developed by Roche Diagnostics, the NEULARK trial has the potential to set a new benchmark for monitoring Parkinson’s disease progression with unprecedented resolution and precision. This cutting-edge technology allows patients to seamlessly incorporate monitoring into their daily lives, yielding additional valuable insights that traditional in-clinic assessments cannot provide.
-
Building Key Collaborations
Our groundbreaking approach to addressing Parkinson’s disease is enabled by our ability to foster key collaborations with industry leaders who share our mission to change the course of PD for patients and the broader community.







 Leveraging Genetics to Identify the Right Patients
Leveraging Genetics to Identify the Right Patients
 Developing Best-in-Class Small Molecules
Developing Best-in-Class Small Molecules
 Innovating in Measuring PD Progression
Innovating in Measuring PD Progression