NEU-411 LRRK2i for PD
We are leveraging our strengths in chemistry, genetic target selection and validation, and novel trial design to uncover a promising new way to fight Parkinson’s disease.

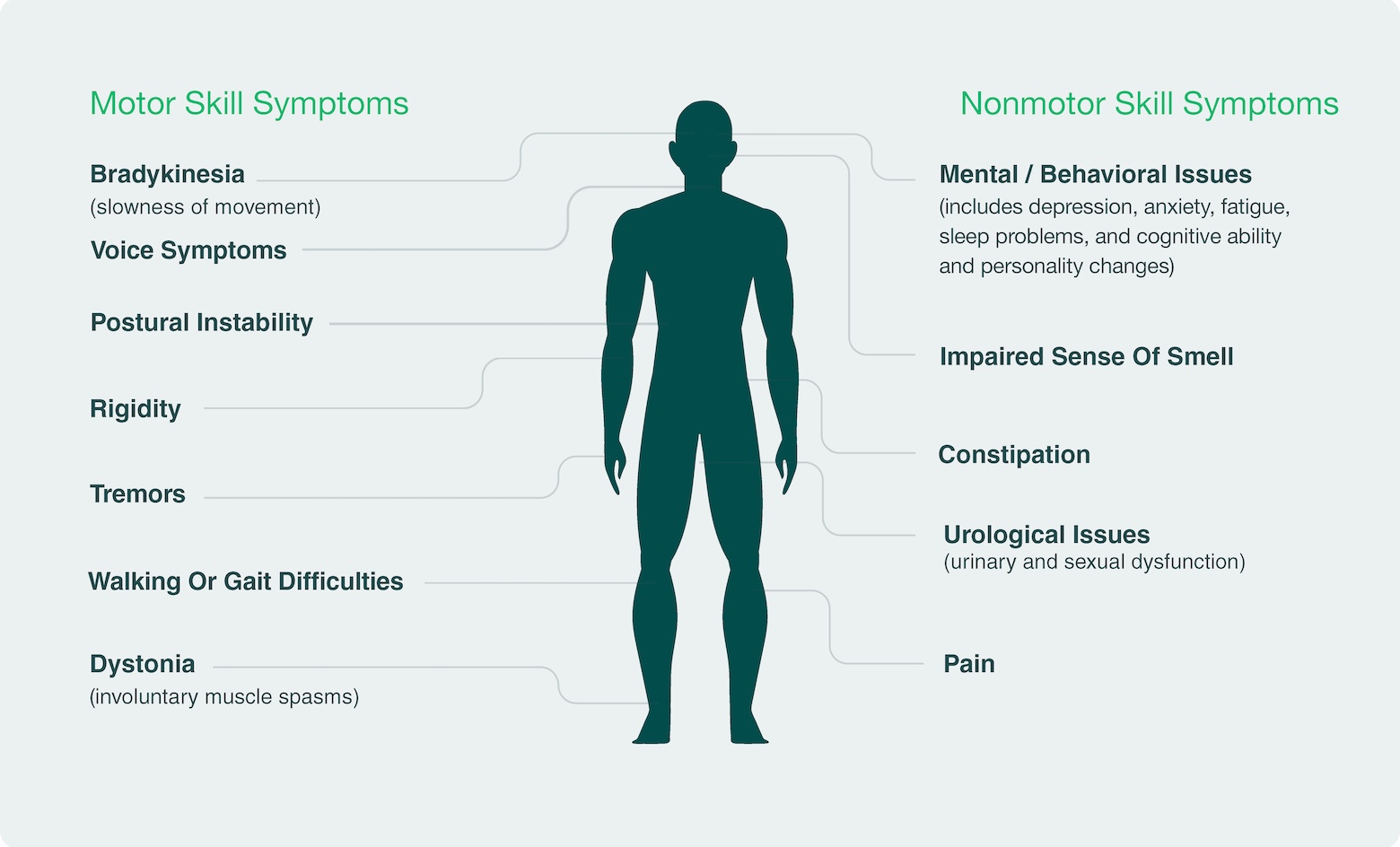
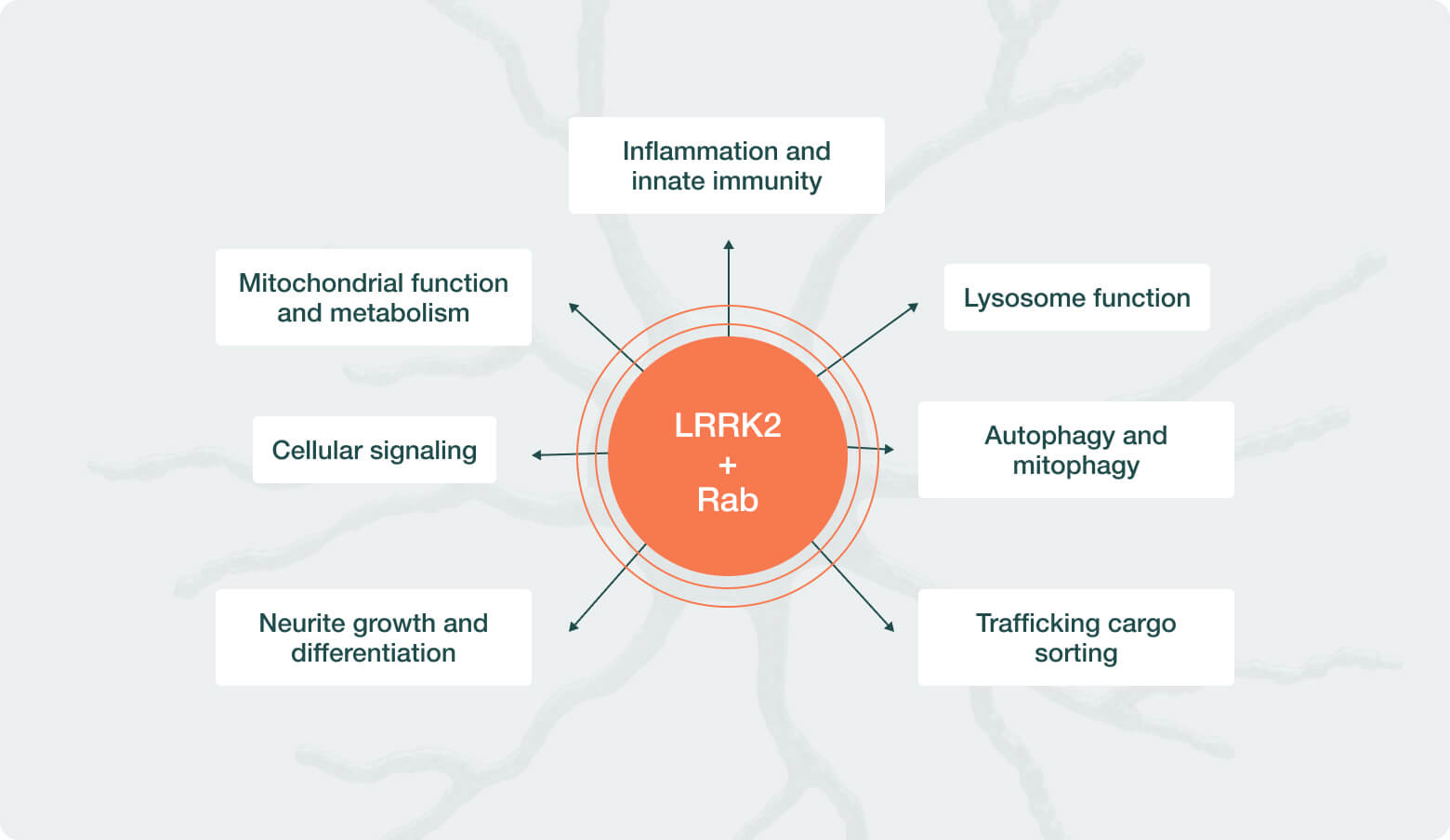
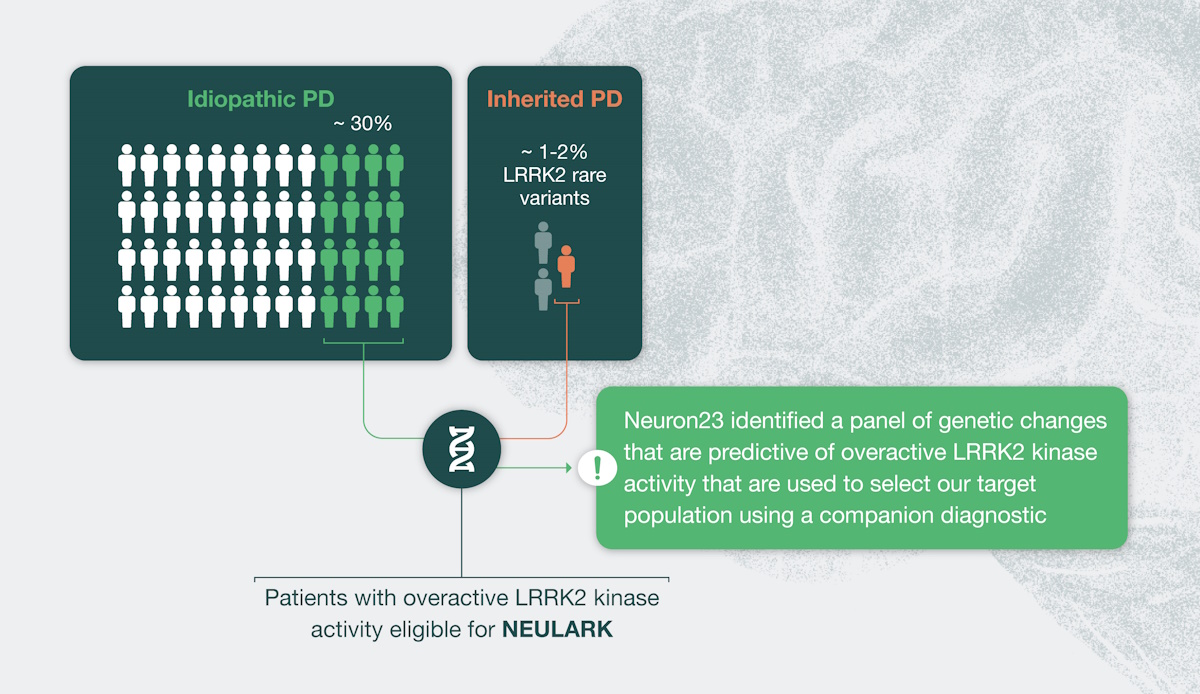
There is emerging evidence that LRRK2 pathway overactivity may be implicated as a driver of Parkinson’s disease (PD) in people with idiopathic PD, or those without a family history of PD. Neuron23 has identified single-nucleotide polymorphisms (SNPs) – common variations or changes in an individual’s DNA – that are predictive of LRRK2 pathway overactivity in over 30% of people with idiopathic PD. People with idiopathic PD who have these SNPs, together with people who have inherited rare LRRK2 gene mutations, make up the population collectively referred to as LRRK2-driven PD and represent the PD patient population predicted to be most likely to benefit from LRRK2 inhibition.

NEU-411 is a brain-penetrant, potent and selective inhibitor of LRRK2 currently being evaluated in the global Phase 2 NEULARK study in people with early Parkinson’s disease. Learn more.
By specifically inhibiting the overactive LRRK2 kinase, NEU-411 aims to address an underlying cause of disease progression in people with LRRK2-driven PD, offering a more precise and potentially more effective approach compared to existing treatment options that only address some symptoms of PD.
The NEULARK study utilizes an investigational next-generation sequencing diagnostic assay developed in collaboration with QIAGEN to select people with LRRK2-driven PD for the trial, representing the first precision medicine approach for people with PD.