Our Science
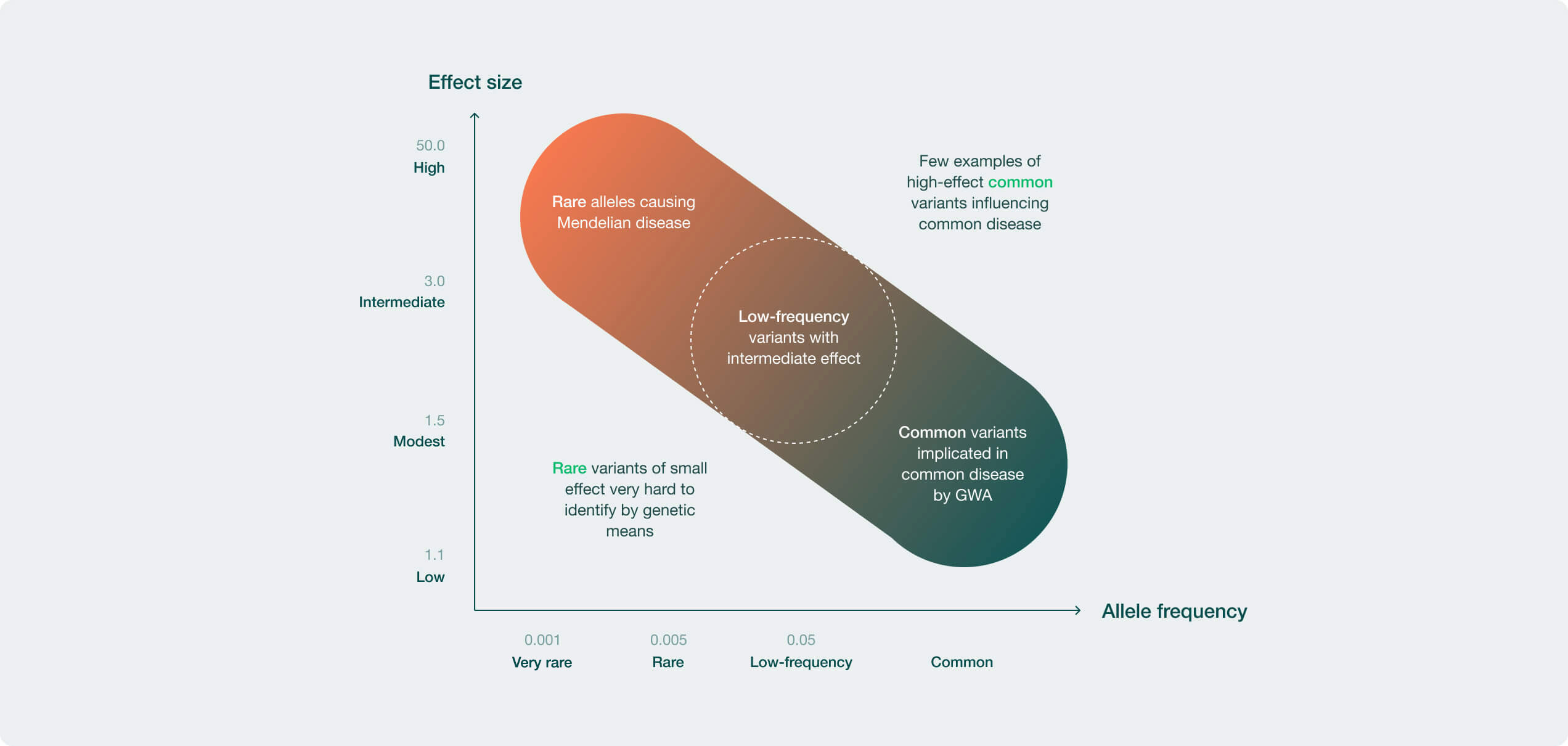
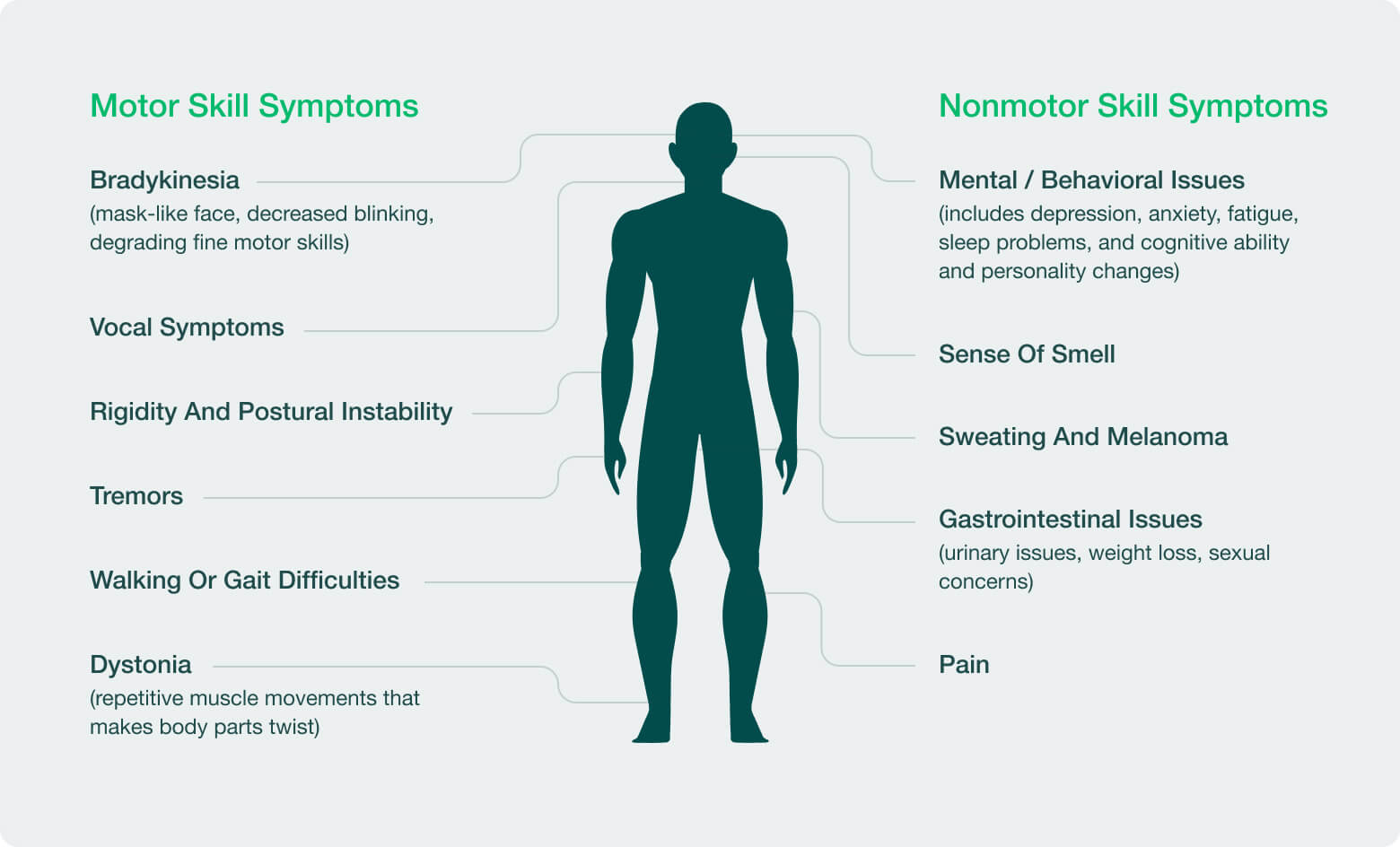
We leverage expertise in human genetics, data science, immunology, and neurology to gain a deeper understanding to the biologic mechanisms and etiology of the diseases we aim to treat and to prioritize the targets within our portfolio.

New Breakthroughs
Require New Methods
We leverage our strengths in chemistry, genetic target selection and validation, and AI-enabled drug discovery to uncover promising new ways to fight disease.
| Target | Therapeutic Area | Discovery | Preclinical | Phase 1 | Phase 2 |
|---|---|---|---|---|---|
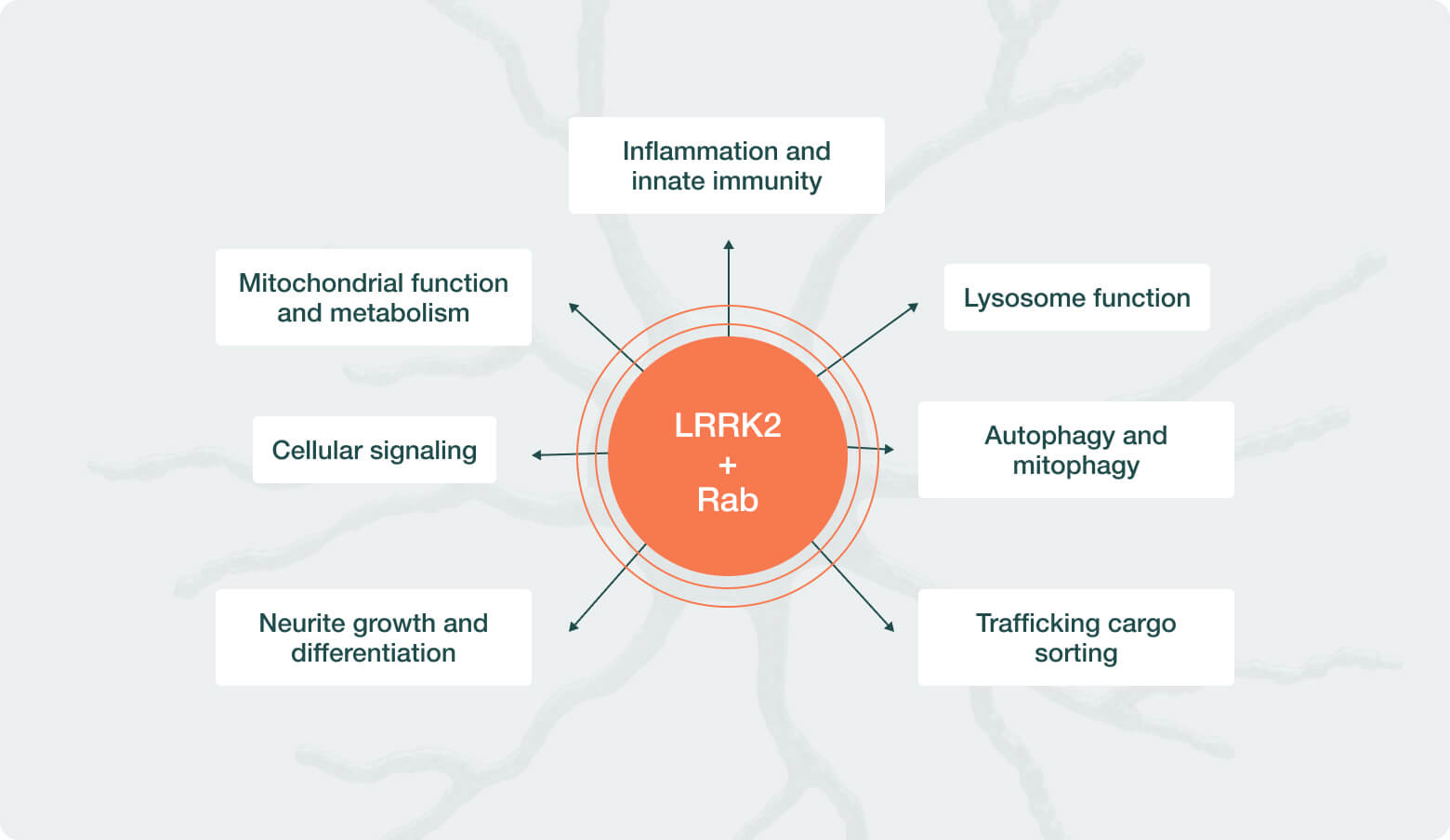
| LRRK2 | Parkinson’s Disease | ||||
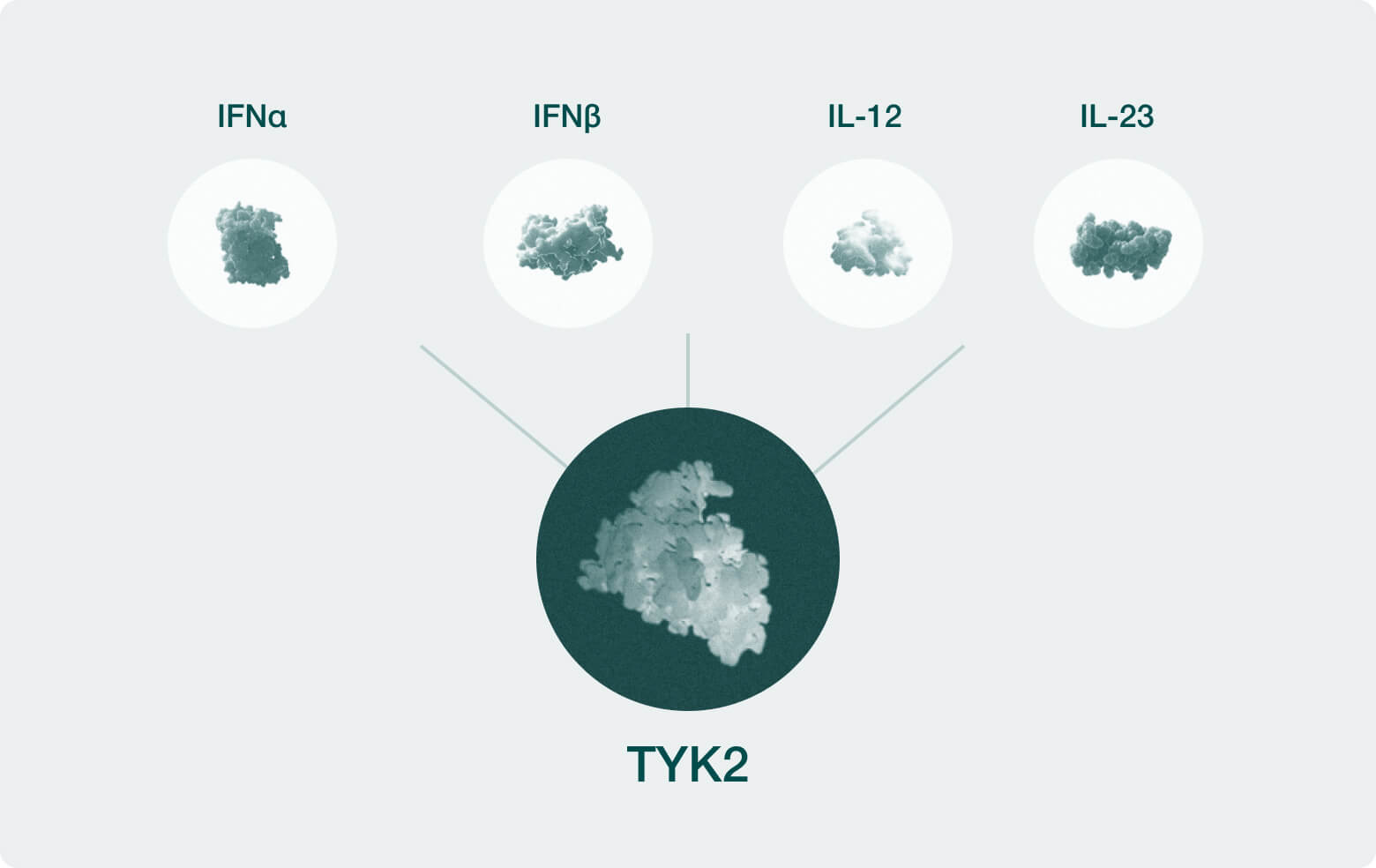
| Systemic TYK2 | Systemic Inflammation | ||||
| Central TYK2 | Neuroinflammation, MS | ||||
| Target 4 | Not disclosed | ||||
| Target 5 | Not disclosed | ||||
This is Just the Beginning
We leverage our strengths in chemistry, genetic target selection and validation, and AI-enabled drug discovery to uncover promising new ways to fight disease.
Neuron23 Series A
Amount raised:
$13M from Kleiner Perkins
Amount raised:
$20.5M from Westlake and Kleiner Perkins
Amount raised:
$80M from Redmile, Cowen, Acorn, Surveyor-Citadel, HBM, Perceptive, Westlake, and Kleiner Perkins
Amount raised:
$100M from SoftBank, Chimera, Redmile Group, Westlake Biopartners, and Kleiner Perkins Caufield and Byers, Cowen Healthcare Investments, Acorn, Surveyor-Citadel, Perceptive Advisors and HBM